
phase separation of dissolved gases as they become more insoluble). As you heat solutions of nonpolar gases in water, the gases become less soluble as evidenced by bubble formation (i.e. Compare the solubility of nonpolar gases like CO2 or N2, which are more soluble at low temperature. they move from the more hydrophobic interior of a protein to the more polar outside). low temperature denaturation of proteins - It has been observed that proteins can denature at low temperatures (less than 0oC), suggesting that nonpolar residues become more "soluble" in water at low temperatures (i.e. (Review free energies of transfer of hydrophobic groups in Chapter 1D: Lipids in Water - Thermodynamics )ī. Studies show that as the surface area of amino acid side chains increase, the free energy of transfer of amino acids from water to ethanol becomes more negative.įigure: Transfer of amino acids from water crystal structures: These structures show that most nonpolar side chains are buried inside a protein, which is tightly packed and which excludes water. If this analogy holds, anything that will promote benzene solubility will lead to increased hydrophobic amino acid side chain exposure to water and hence protein denaturation.
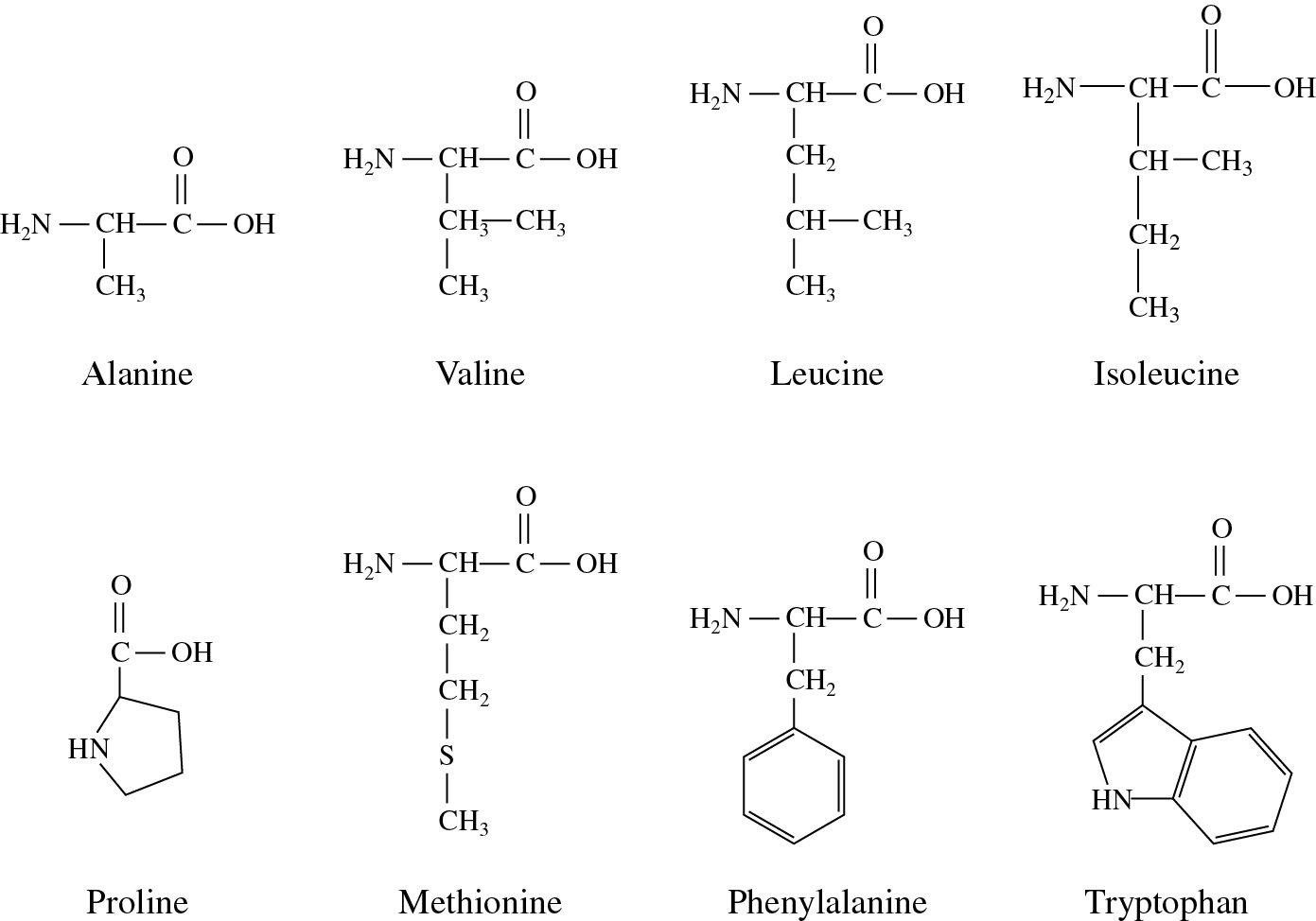
Does this also drive protein folding? To explore this questions, we will study the thermodynamics of small nonpolar molecules, especially benzene, with water and ask whether the thermodynamic parameter associated with benzene solubility are similar to those associated with protein stability. We have studied the role of the hydrophobic effect (involving the favorable entropic release of caged water molecules about solvent-exposed hydrophobic groups) in driving micelle and bilayer formation.


 0 kommentar(er)
0 kommentar(er)
